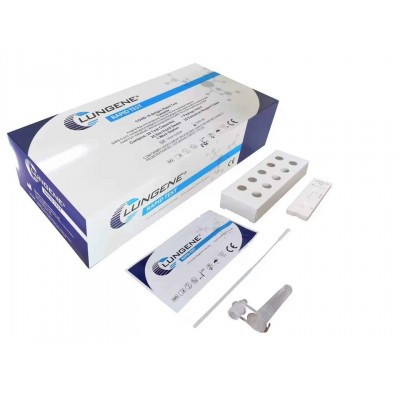
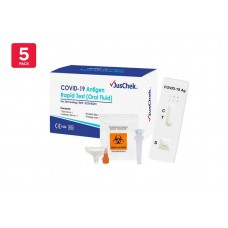
Rapid Antigen Test - Nasal Swab/ Nasal Test Clungene COVID-19 Antigen Test Cassette TGA Approved PICK UP ONLY
Rapid Antigen Test - Nasal Swab/ Nasal Test Clungene COVID-19 Antigen Test Cassette TGA Approved PICK UP ONLY
- Product Code: Clungene
- Availability: 100
- SKU: SMZHCOVID5N
Price
-
$11.00
- Ex Tax: $10.00
Order NowNo returns once ordered.Only till stock lasts
Rapid Antigen Test - Nasal Swab/ Nasal Test Clungene COVID-19 Antigen Test Cassette TGA Approved PICK UP ONLY
|
|
When only the best will do! Another Quality Product you can trust from Solutions Medical Quality - Precision - Technology at Competitive Prices!!!
FEATURES:
All our products are included in the Australian Register of Therapeutic Goods (TGA) register. Clungene COVID-19 Rapid Antigen Test for Home, Set of 5 Applications, Nasal Sampling
INTENDED USE The SARS-CoV-2 Antigen Rapid Test (Nasal Swab) is a lateral flow chromatographic immunoassay single-use test kit intended to qualitative detect the SARS-CoV-2 that causes COVID-19 with self-collected nasal swab specimen. The test is intended for use in symptomatic individuals meeting the case definition for COVID-19 within the first 7 days of symptom onset. Results are for the detection of SARS-CoV-2 Nucleocapsid protein Antigens. An antigen is generally detectable in upper respiratory specimens during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results are indicative of the presence of SARS-CoV-2. Individuals who test positive should self-isolate and contact your State or Territory Coronavirus testing services to get a laboratory PCR test. Positive results do not rule out bacterial infection or co-infection with other viruses. Negative results do not preclude SARS-CoV-2 infection. Individuals who test negative and continue to experience COVID-like symptoms should seek follow up care from their COVID testing centre. he SARS-CoV-2 Antigen Rapid Test (Nasal Swab) obtain a preliminary result only, an aid diagnosis of COVID-19, for the final confirmation should be based on clinical diagnostic results. SUMMARY The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhoea are found in a few cases1. PRINCIPLE The SARS-CoV-2 Antigen Rapid Test (Nasal Swab) is a qualitative membrane-based immunoassay for the detection of SARS-CoV-2 nucleocapsid protein antigens in human swab specimen PRECAUTIONS Please read all the information in this package insert before performing the test. • For self-testing in vitro diagnostic use only.Do not use after expiration date. • This test kit is intended to be used as a preliminary test only and repeatedly abnormal results should be STORAGE AND STABILITY Store as packaged in the sealed pouch at room temperature or refrigerated (2-30 °C). The test is stable through the expiration date printed on the sealed pouch. The test must remain in the sealed pouch until use. DO NOT FREEZE. Do not use beyond the expiration date. VIDEO:https://youtu.be/hCejITR8_zY Frequently Asked QuestionsHow does the Covid-19 Antigen Rapid test ( Self Test ) work? Is this kit TGA approved? What age group can be tested on Covid-19 Antigen Rapid Self testing device? Anyone above the age 2 can be tested. For children younger than 14 years, an adult caretaker should help collect the nasal samples and conduct the test procedures. What does positive test result means for me? What does negative test result means for me? What does an invalid result mean? What are the advantages of performing self testing? How does the nasal swab work? The nasal swab head has a brush which trap the virus in its bristles when the swab is rotated in the nostrils brushing it against the walls of the nasal passage. When the swab is inserted in the buffer and squeezed, the virus breaks down and is released in the buffer. Why do I need to swab both nostrils? How do I collect sample of a child? If I have a runny or blocked nose can I use the swab to do the test? Information regarding available support services can also be obtained by contacting your local state and territory health department at: Australian Capital Territory Department of Health 02 62077244 https://www.health.act.gov.au/ New South Wales Department of Health 137788 https://www.health.nsw.gov.au/ Northern Territory Department of health 1800020080 https://www.health.nt.gov.au/ Queensland Department of health 134268 https://www.health.qld.gov.au/ South Australian Department of Health 1800253787 https://www.sahealth.sa.gov.au/ Tasmanian Department of Health 1800671738 https://www.health.tas.gov.au/ Victorian Department of Health 1800675398 https://www.dhhs.vic.gov.au/ Western Australian Department of Health 1800595206 https://www.healthywa.wa.gov.au/ If there are poor performance or usability issues: Use Medical Device incident Report Call 1800809361 or E-mail [email protected] |
Related Products
Grab 'N Pull Seatbelt Reacher Reduces Strain Pain Arthritis and Back Issues
$22.00 Ex Tax: $20.00
Alcocheck Personal Breath Scan Alcohol Check (0.05)
$5.50 Ex Tax: $5.00
Sterile Splinter Probes - First Aid (10 Pieces)
$3.85 Ex Tax: $3.50

 AUSTRALIA-WIDE SHIPPING $15
AUSTRALIA-WIDE SHIPPING $15